More lot release of vaccines will help meet production demand

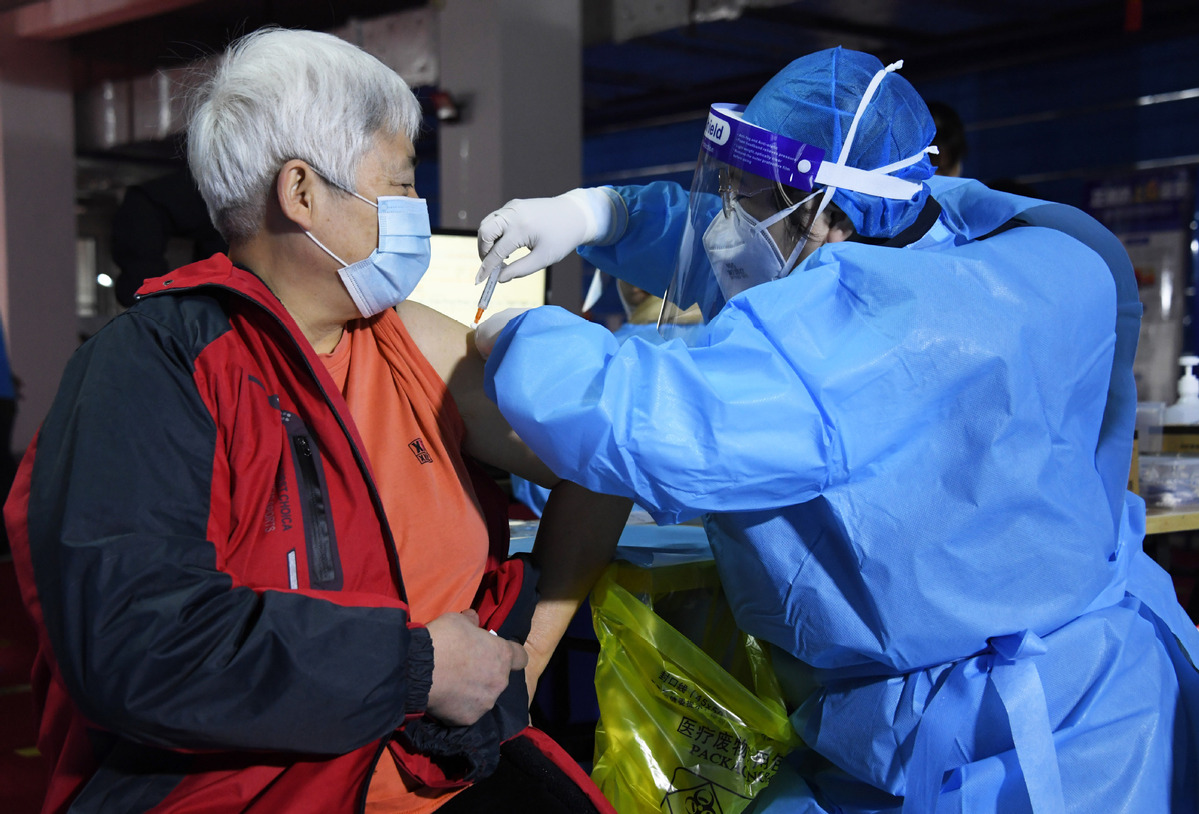
About a dozen provincial medical product inspection institutions in China will soon be capable of conducting the lot release of vaccines to meet the demand of COVID-19 vaccine production in the country, a health official said on Monday.
Yuan Lin, an official at the National Medical Products Administration, said apart from a national medical product inspection institution, another three, in Beijing, Hubei and Guangdong, also can conduct the inspection and lot release of COVID-19 vaccines.
The country is actively promoting the lot release and inspection capability in provincial institutions, he said.
Vaccines are key medical products that require stringent supervision, he said.
The vaccine management law, which came into effect in December 2019, stipulates that each individual lot of vaccines, domestic or imported, must be evaluated by State-approved institutions before being approved for market, Yuan said at a news conference.
- Liaison office of central govt spokesperson hails Macao SAR 8th Legislative Assembly election
- China discovers highest-altitude Qin Dynasty engraved stone on Qinghai-Tibet Plateau
- Traditional culture enthusiasts experience handicraft exchange at museum in China's Xinjiang
- Promoting intl rule of law for fairer global governance
- Sovereign equality is anchor of fairer global governance
- Xi's vision for better global governance seeks to build a fairer world