Initial clinical trial shows CoronaVac vaccine safe for children, adolescents: study

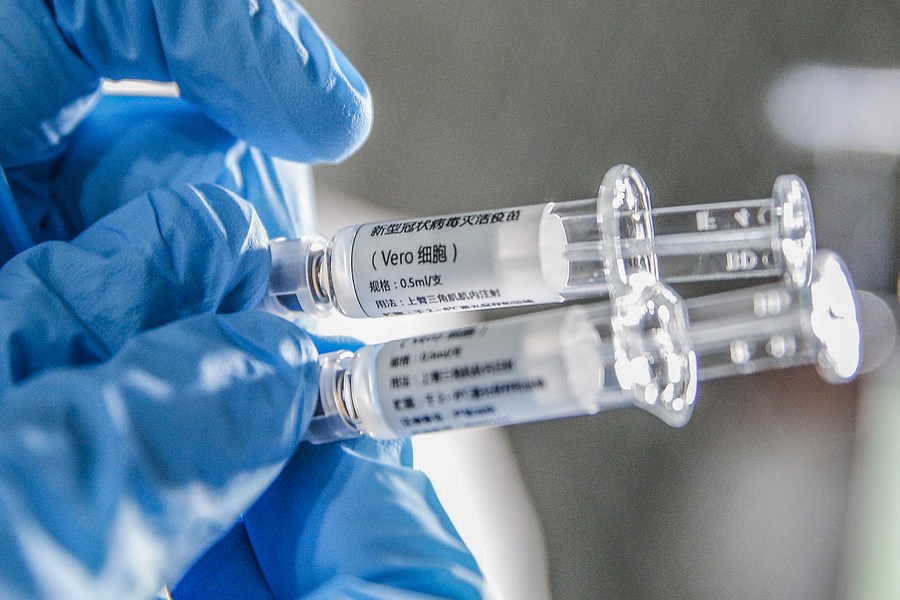
LONDON - Phase 1/2 clinical trial of the CoronaVac COVID-19 vaccine in over 500 children and adolescents suggests two doses of the vaccine are safe and generate a strong antibody response, according to a study published Monday in The Lancet Infectious Diseases journal.
Chinese researchers conducted a randomized, double-blind, controlled phase 1/2 clinical trial of the CoronaVac COVID-19 vaccine, developed by Chinese pharmaceutical company Sinovac Biotech, in more than 500 healthy children and adolescents aged three to 17 years in China.
The results showed that more than 96 percent of children and adolescents who received two doses of the vaccine in the trial developed antibodies against the novel coronavirus (SARS-CoV-2), the study said.
Most adverse reactions were mild or moderate, with pain at the injection site, the most commonly reported symptom, according to the study.
But the researchers also said that, owing to the small number of participants in the study, the results should be interpreted with caution as it was not possible to draw strong statistical conclusions.